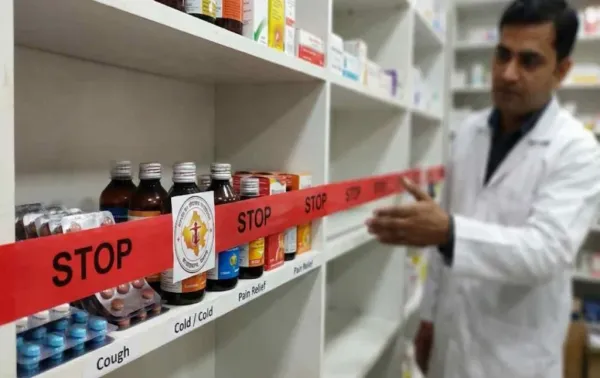
In Rajasthan, 16 medicines did not meet the quality standards. These include medicines for cough, cold, fever and vitamins. The State Commissionerate ordered medical stores to stop their sale and started preparations for action against the pharmaceutical companies.
Rajasthan: The Food Safety and Drug Control Commissionerate collected samples of 16 medicines from different districts of the state in the first fortnight of January 2026. Lab reports found serious flaws in many medicines. The active ingredients in some medicines were found to be less than the prescribed quantity, while some could not even meet the standards of the Indian Drug Code (IP). Therefore these medicines were declared NSQ i.e. ‘Not of Standard Quality’. NSQ medicines are neither safe nor effective in treatment.
Which medicines are included
Medicines used daily in the market are also included in this list. These include calcium and vitamin D3 tablets, fever and pain medicine paracetamol, cough and cold medicines such as ambroxol, terbutaline and guaifenesin. Apart from this, some antibiotics and blood pressure medicines were also found to have failed.
The Commissionerate has ordered all medical stores and drug sellers to stop the sale of these medicines immediately. Also, preparations are being made to take action against the concerned pharmaceutical companies and sellers under the Drugs and Cosmetics Act, 1940 and Rules 1945.
Danger to patients
Officials say that poor quality medicines can pose a serious threat to patients. If medicine is not given in the prescribed quantity, the treatment is not effective and the disease may worsen. This is the reason why regular testing and strict monitoring of medicines is considered necessary.
To ensure the quality of medicines in Rajasthan, the Commissionerate is constantly monitoring the market. Immediate removal of NSQ declared medicines is important for patient safety.
what is nsq
NSQ means “Not of Standard Quality”, i.e. the medicine which does not meet the prescribed standards and quality. When a medicine contains less than the prescribed amount of active ingredients, does not follow the rules of the Indian Drug Code (IP), or contains impurities, it is considered NSQ. Such medicines are not safe and are not effective in treatment.
If the patient does not receive the right amount of medicine, the disease cannot be cured and may have serious effects on health. Therefore, it is necessary to immediately remove NSQ medicines from the market. State and central government agencies conduct regular checks and take strict action against substandard medicines.